Sample from the Workplace
When I asked Mr. Tam if he had some kind of sample from his work that would help show a relationship between his job and our chemistry curriculum, he immediately thought of some of the basic calculations that he uses everyday as a pharmacist. Recalling the difficult examinations he prepared for that were necessary for him to pass in order to become a licensed pharmacist, he lent me one of his preparatory study booklets, saying that the problems in there were some of the best examples of questions that he dealt with most often in his job. Below are two of the problems from this booklet, explained.
Example #1:
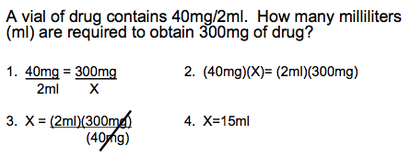
This problem is a typical question that appears quite often in the life of a pharmacist. It contains simple calculations, taught in the Grade 11 Chemistry curriculum. Stoichiometry is used to solve this problem. In this case, Mr. Tam was asked to solve for the amount of ml required to obtain 300mg of a certain drug. Setting up a simple mathematical and stoichiometric equation, he quickly solved for value X, which was designated as the amount of the drug in ml.
Example #2:
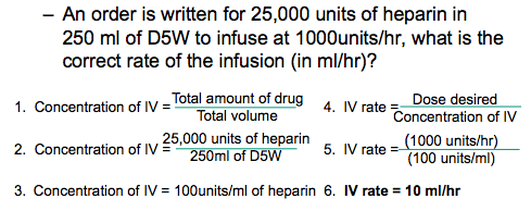
Example #2 is another type of question Mr. Tam solves quite regularly. It involves the Grade 11 Chemistry concept of concentration. Concentration is generally defined as the amount of solute per the total volume. In this case, the concentration of IV is equivalent to the total amount of drug per the total volume. After plugging the correct values into this equation, Mr. Tam then subbed his answer into a second equation; the equation for IV rate (dose desire per concentration of IV). This allowed him to arrive at his final answer.
